Global Journal of Medical and Clinical Case Reports
Influence of Additives on Crystallization of Calcium Phosphates from Prototypes of Blood Plasma
Golovanova OA*
Dostoevsky Omsk State University, Omsk, Russia
Cite this as
Golovanova OA. Influence of Additives on Crystallization of Calcium Phosphates from Prototypes of Blood Plasma. Glob J Medical Clin Case Rep. 2024:11(4): 030-033. Available from: 10.17352/2455-5282.000183Copyright License
© 2024 Golovanova OA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The paper presents the results of studies on the effect of additives on crystallization in solutions simulating the composition of human blood plasma. Synthesis from prototypes of human blood plasma in the presence of organic and inorganic additives was carried out, and it was found that the obtained solid phases consisted of octacalcium phosphate, B-type carbonate hydroxyapatite, and vitlocite. The effect of additives (magnesium ions, alanine, and glycine) on the crystallization of calcium phosphates was studied. It was found that the presence of additives in the model solution reduces the crystallite size and the fraction of carbonate hydroxyapatite in the solid phase. The bioactivity of synthetic samples was studied, and kinetic characteristics were established. A study of thermal transformations.
Introduction
Currently, the percentage of pathogenic mineral formation in blood vessels and heart valves has increased [1-4]. This is due to a number of factors of both exogenous and endogenous nature. Therefore, the study of crystallization processes of poorly soluble compounds formed in the human body for the purpose of disease prevention and warning is a promising area of research. Blood plasma is water containing mainly dissolved salts and proteins. Soluble substances in plasma make up about 10% of the blood mass, of which about 7% are proteins, 0.9% are inorganic salts, and the rest are non-protein organic compounds [5].
According to the results of a study of coronary artery lesions, the authors of [6] identified three independent phases of atherosclerotic lesions of the coronary arteries associated with calcification of atherosclerotic plaques: a) fibrous plaque sites with low lipid content; b) the final stage of the formation of necrotic foci; c) hemorrhage and thrombosis.
Calcifications are typical biogenic minerals, closely associated spatially, genetically structurally, and morphologically with the components of body tissues. The degree of defectiveness is always high and depends more on local processes than on the state of the organism as a whole. The Ca/P ratio in calcinates, according to published data, varies significantly [7, 8]. In matrix vesicles, this ratio can be about 0.66, and in mature apatite, it reaches 2. The crystal size during the maturation of bioapatite increases from nanoscale to 100 and even more microns. Pathogenic apatite does not have such a close connection with metabolic processes in the body as physiogenic. From this, we can conclude that the crystallochemistry of natural inorganic apatite is incredibly complex, and the structure of bioapatite has a number of additional features, while the mineralization of tissues has been studied insufficiently [9-11].
It is because of calcification that the duration of heart valve transplants is limited [12-14]. In this regard, there is a need to study the mechanism of calcification initiation of both collagen and muscle tissues and heart valve transplants in order to develop ways to prevent it based on the obtained ideas.
The purpose of the work is to study the crystallization of calcium phosphates from prototypes of human blood plasma in the presence of inorganic and organic additives, to study their properties (bioactivity, thermal degradation).
Materials and methods
To calculate the composition of model solutions, the mean concentration of inorganic ions entering the human blood plasma was used [15]. The choice of initial reagents and their ratio in the solution was determined so that the ion concentration and ionic strength of the solution were as close as possible to the parameters of human blood plasma. The initial reagents were pure and chemically pure salts and distilled water. For each series of experiments, solutions were prepared containing cations and anions, the combined presence of which under the given conditions does not result in the formation of poorly soluble compounds. In each, the pH values were adjusted to the physiological value (7.4 ± 0.01) by adding a 30% solution of NaOH or HCl. After mixing equivalent volumes of the solutions, we obtain a solution with a given supersaturation and the calculated concentration of the components. At the end of the synthesis time, the solution is filtered. A portion of the supernatant is collected for chemical analysis. After filtration, the precipitate is dried in a drying cabinet at a temperature of ~ 80 °C until all water is removed.
To study the effect of inorganic and organic substances, the corresponding components were added to the model system: magnesium ions in concentrations exceeding the physiological 2, 4 times; glycine, alanine - in physiological concentrations and exceeding physiological 10 and 50 times (Table 1).
The phase composition of the obtained precipitates was studied using X-ray powder diffraction (DRON-3) and IR spectroscopy (FT-02 spectrophotometer). The peaks in the diffraction patterns were identified using the JCPDS card file and the DifWin 4.0 and Crystallographica Search-Match software packages. The content of the phases present in the samples was determined by the corundum number method (Chang method, Crystallographica Search-Match program). The sizes of the coherent scattering region (CSR, minimum crystallite sizes) of solid phases are determined by the Debye – Scherrer formula. The parameters and volume of the unit cell of the crystals were calculated by the formulas for the hexagonal HA syngony.
To study the energy of the processes of thermal decomposition of the obtained solid phases, thermogravimetric analysis (TGA) was carried out. A test sample weighing 0.2000 ± 0.0005 g was placed in a ceramic crucible and heated in a muffle furnace from 200 ⁰C to 1000 ⁰C, with step 200 ⁰C.
The resorption (solubility) of the samples was studied by dynamically dissolving them with constant stirring in a solution of 0.9% sodium chloride (pH 7), in acetate (pH = 4.75) and Tris buffer (pH = pH = 7 4) at a temperature of 18 - 20 0 C. Based on the obtained experimental data, kinetic curves were obtained and their mathematical processing was carried out according to the algorithm described in the work.
Results and discussion
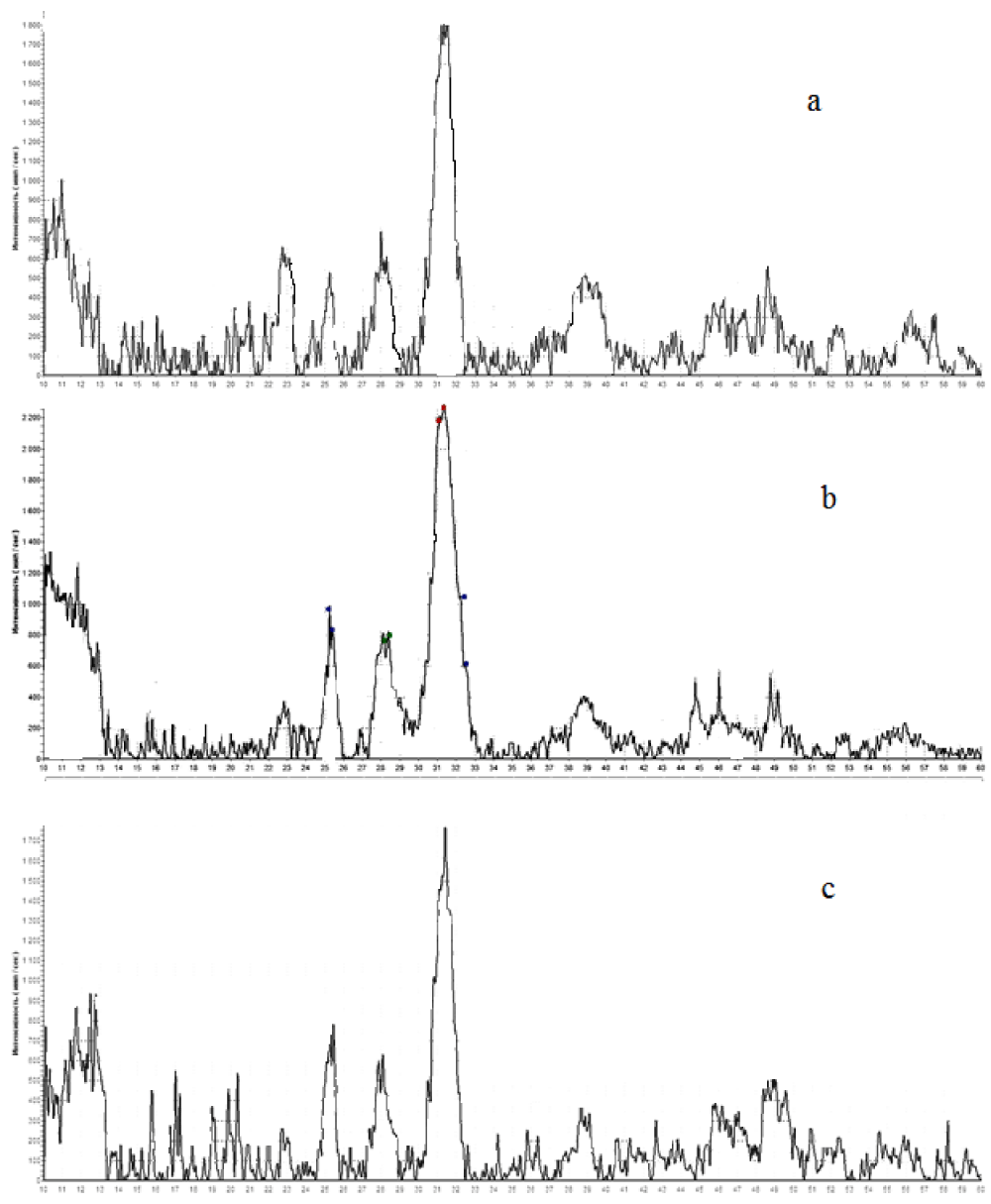
X-ray powder diffraction analysis showed that all samples synthesized in a model blood plasma solution with varying supersaturations and synthesis times are a mixture of Hydroxylapatite (НA), Octocalcium Phosphate (OCF), and vitlocite (Figure 1). OKF lines correspond to peaks on a 2θ scale: 28.3; 34.4; 36.7, KНA lines correspond to peaks on a 2θ scale: 26.3; 33.9; 34.6, and the lines of vitlocite correspond to peaks on a 2θ scale: 17.4; 30.2; 33.7.
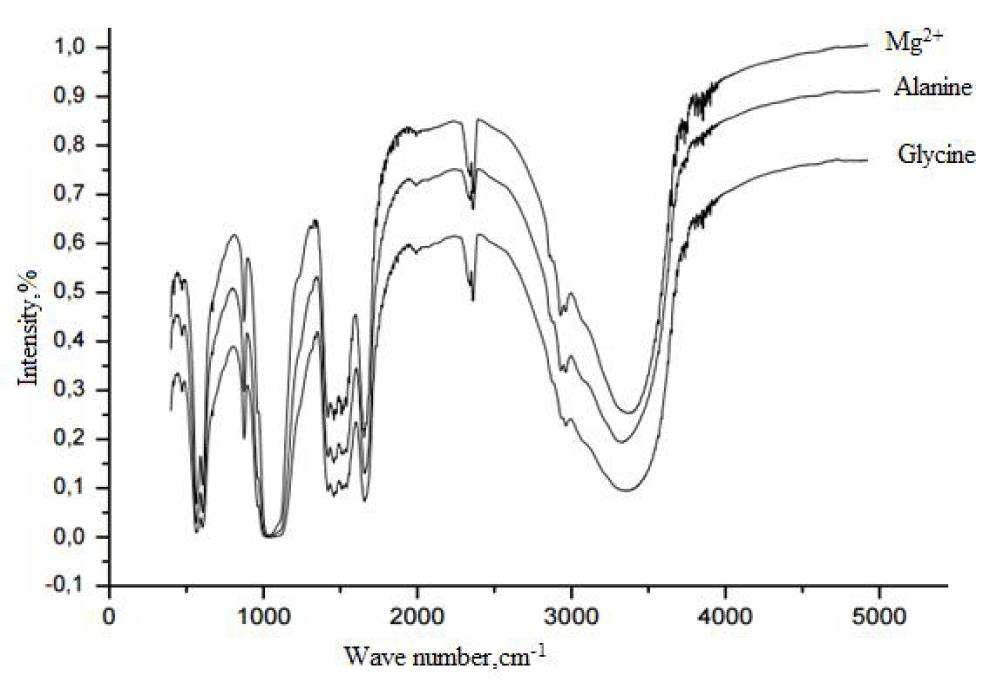
It was found that in the IR spectrum, (Figure 2) of all the studied samples in the region of 1000 - 1200 cm-1 the stretching asymmetric vibrations of the P-O bonds of the PO4 3- ion appear in the region of 920 - 980 cm-1 - symmetric stretching vibrations of the P- bonds O ion PO4 3- in the region of 500 - 650 cm-1 - deformation asymmetric vibrations of the P-O bonds in the PO4-3 ion. Also, in the IR spectra of the samples, absorption bands are recorded in the spectral regions of 1400 - 1500 and 850 - 900 cm-1, which are related to stretching and deformation vibrations of C – O bonds of carbonate ions, respectively. It can be seen that the intensity characteristic for hydroxylapatite bands of OH vibrations is very low in the region of 3500 cm-1. From this, we can conclude that the precipitate contains B-type carbonate hydroxylapatite.
It was found that the presence of additives in the initial model solution of human blood plasma reduces the crystallite size (Table 2).
The size of crystallites decreases with increasing supersaturation. In our opinion, organic additives can have a bilateral effect on crystallite size. One of the options is the adsorption of molecules of organic additives on the faces of the formed crystallites and the poisoning of their growth. The second option is the formation of complex compounds with calcium ions in solution. When administration and a solution of magnesium ions crystallite size is significantly reduced. This may be due to a slowdown in the formation of calcium phosphates in the presence of magnesium ions, as well as to an insignificant formation of a sparingly soluble compound Mg3 (PO4)2. Having analyzed the obtained results, we can propose a number of additives that affect the crystallite size of the solid phase: Mg2+ Kinetic data on the bioactivity of the obtained samples were processed and the reaction order was determined by the graphical method. For each sample, it was equal to zero, which is characteristic of heterogeneous ion exchange reactions occurring in solution. Also, for each sample was determined by the speed of dissolution and found that, with increasing supersaturation dissolution rate does not vary monotonically, the difference is explained, and the change in the phase composition of solid powders [16,17]. Analysis of the time dependence of the samples under study did not reveal general trends for the two solvents. It was found that the highest dissolution rate of the samples was recorded in an acetate buffer solution, in our opinion, this is due to the acidic environment of this solution (Table 3). The main processes occurring during the heat treatment of the synthesized samples in the temperature range of 200 °C -800 °C are estimated. It was established that in the temperature range from 200 °C – 400 °C, the greatest decrease in mass is observed, which is consistent with the data [6]. This decrease is associated with the removal of adsorption and crystallization water. In the range from temperatures of 600 °C - 800 °C, carbonate ions are removed from the structure of carbonate hydroxylapatite. The main phase transformations of crystallization products as a result of heat treatment are presented in Table 4. The shape of crystallites after heat treatment is shown in Figure 3. As a result of the work performed, the process of crystallization from human blood plasma prototypes was studied and the properties of the obtained solid phases were determined. Based on the results of the work, the following conclusions can be drawn:Conclusion
- Golovanova OA, Frank-Kamenetskaya OV, Punin YO. Specific features of pathogenic mineral formation in the human body. Russ J Gen Chem. 2011;81(6):1392-1406. Available from: http://dx.doi.org/10.1134/S1070363211060442
- Kodaka T, Mori R, Hirayama A, Sano T. Fine structure and mineral components of fibrous stonelike masses obtained from the human mesenteries. The Clin Electron Microsc Soc Japan. 2003;36:272-278. Available from: http://dx.doi.org/10.1007/s00795-003-0234-z
- Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2015;13(2):1038. Available from: https://doi.org/10.1038/nrcardio.2015.164
- Olsson LF, Sandin K, Odselius R, Kloo L. In vitro formation of nanocrystalline carbonate apatite—a structural and morphological analogue of atherosclerotic plaques. Eur J Inorg Chem. 2007:4123-4127. Available from: https://lup.lub.lu.se/search/publication/46df2104-d925-4d91-8992-abe713026416
- Lamanova LM. Tissue calcification in the cardiovascular system. Bull TSU. 2010;337:194-197.
- Golovanova OA, Kutuzova YA. The study of the characteristics of calcium phosphates synthesized from model solutions of blood plasma. Bull Omsk Univ. 2016;(1):56-62.
- Izmailov RR, Golovanova OA. Bioresorbability of a granular composite based on carbonate hydroxylapatite and gelatin in media with different pH values. Tomsk State Univ J. 2015;(2):61-5.
- Golovanova OA. Pathogenic minerals in the human body. Omsk: Publishing House of Omsk State University. 2006; 400.
- Iwaki R, Shoji T, Matsuzaki Y, Ulziibayar A, Shinoka T. Current status of developing tissue engineering vascular technologies. Expert Opin Biol Ther. 2022;22:433-440. Available from: https://doi.org/10.1080/14712598.2021.1960976
- Naegeli KM, Kural MH, Li Y, Wang J, Hugentobler EA, Niklason LE. Bioengineering human tissues and the future of vascular replacement. Circ Res. 2022;131(2):109-126. Available from: https://doi.org/10.1161/circresaha.121.319984
- Ketelhuth DF, Lutgens E, Bäck M, Binder CJ, Van den Bossche J, Daniel C, et al. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res. 2019;115(9):1385-1392. Available from: https://doi.org/10.1093/cvr/cvz166
- Silvis MJ, Demkes EJ, Fiolet AT, Dekker M, Bosch L, van Hout GP, et al. Immunomodulation of the NLRP3 inflammasome in atherosclerosis, coronary artery disease, and acute myocardial infarction. J Cardiovasc Transl Res. 2021;14(1):23-34. Available from: https://doi.org/10.1007/s12265-020-10049-w
- Yakhno T. Protein phase instability developed in plasma of sick patients: clinical observations and model experiments. Nat Sci. 2010;3:220-227. Available from: http://dx.doi.org/10.4236/ns.2010.23034
- Ambrosi D, Quarteroni A, Rozza G, editors. Modeling of physiological flows. Springer-Verlag Italia. 2012; 418. Available from: https://link.springer.com/book/10.1007/978-88-470-1935-5
- Pukhov DE, Vasilev SV, Zotov AS, Rudy AS. Localization habits and composition of mineral deposits in atherosclerotic plaques of coronary arteries according to the scanning electron microscopy and X-ray diffractometer. 2014;49.
- Maslakov DA, Khodosovsky MN, editors. Pathophysiology of the blood system. Grodno: GGMU. 2004; 52.
- Chignon A, Bon-Baret V, Boulanger MC, Bossé Y, Mathieu P. Arterioscler Thromb Vasc Biol. 2021;41:11. Available from: https://doi.org/10.1016/j.isci.2021.102241